TRANSCENTA HOLDING - A Global Fully Integrated Biotherapeutics Company
Transcenta, Biologics, Antibody, Claudin 18.2

In The News
 2025 - 03 - 04
2025 - 03 - 04
Founded in 2012 around the company’s immune tolerance-breaking (IMTB) technology platform, Transcenta is a clinical stage biopharmaceutical company creating antibody-based therapeutics. The company’s headquarters, research and development (R&D) and manufacturing sites are in China, with a clinical research and external partnering center in the US.
“By carrying out clinical development globally, we can see how our therapeutics work in different populations, helping us to advance our pipeline to meet unmet needs in patients worldwide,” said Xueming Qian, founder, chair and CEO of Transcenta.
Using its IMTB platform, Transcenta can generate antibodies—with broad epitope diversity and enhanced druggability—to conserved and non-conserved proteins, and discover hidden epitopes. The lead candidates are screened using in-house platforms, and companion diagnostics are created to aid stratification for clinical trials and support therapeutic use following approval. Transcenta’s experienced chemistry, manufacturing and controls (CMC) team uses its technology platforms to develop and formulate candidates for clinical trials.
Transcenta’s pipeline has 14 therapeutic antibodies with applications in oncology, nephrology, bone health and the treatment of other disorders. The lead molecule, osemitamab (TST001), is a second-generation, humanized, high-affinity antibody that targets the transmembrane protein Claudin-18.2, expressed in a range of solid tumors (Fig. 1). Osemitamab has been engineered to obtain enhanced antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).
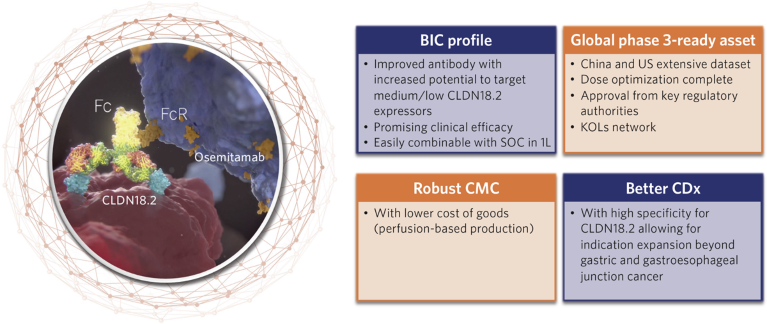
Fig. 1 | Osemitamab (TST001). The best-in-class (BIC) anti-CLDN18.2 antibody with a differentiated profile and stronger anti-tumor activity. CMC, chemistry, manufacturing and controls; CDx, companion diagnostics; KOL, key opinion leader; SOC, standard of care.
“Gastric cancer [GC] has a high prevalence across east Asia, with around 60% of cases occurring in this region and 40–45% in China alone,” said Qian. “The global market could be worth multi billions.”
Phase 3 clinical trials of osemitamab in GC and gastroesophageal junction cancer (GEJC) in combination with chemotherapy and PD1 inhibitor are planned, with dosing beginning in 2025 in the US, Europe and China.
“Chemotherapy combined with immune checkpoint inhibitor offers a significant clinical benefit for patients with GC, and it has been approved in the US for this indication. We believe that adding osemitamab in appropriate patients will improve outcomes even further,” said Qian. “In our phase 2 trial, Claudin-18.2 medium-/high-expressing patients had a median progression-free survival of 14.2 months, and more than 70% were alive at 1 year, as reported at ESMO [the European Society for Medical Oncology Congress] 2024.”
Transcenta has an ongoing global clinical collaboration with Bristol-Myers Squibb to evaluate osemitamab in combination with nivolumab for first-line GC/GEJC in phase 2 trials. Clinical trials of osemitamab in combination with chemotherapy as a first-line treatment in GC/GEJC and pancreatic ductal adenocarcinoma (PADC) are also underway, and the agent may have potential as a maintenance therapy post-surgery for GC, and in the treatment of biliary and lung cancers.
Transcenta is working on a Claudin-18.2 companion diagnostic for use in clinical trials and on the market, in collaboration with Agilent Technologies.
TST003 is a first-in-class high-affinity antibody targeting Gremlin-1, which is upregulated in a range of solid tumors, including those resistant to checkpoint inhibitors.
“It has historically been difficult to develop antibodies against Gremlin-1, but we were able to do so using our IMTB platform. We have completed dose escalation in a phase 1 trial in the US and China in locally advanced or metastatic solid tumors, and the next step will be to combine TST003 with standard-of-care in selected tumors,” said Qian. “We believe that TST003 may also have potential in combination with osemitamab.”
The company has two antibody–drug conjugates, TST013 (anti-LIV1) and TST105 (anti-FGFR2b), in preclinical development for a number of different solid tumors. It also has candidates in development for autoimmune disease, including TST004, an antibody targeting mannan-binding lectin serine protease 2 (MASP2) for immunoglobulin A nephropathy (IgAN) and thrombotic microangiopathy; TST801, a second-generation anti-BAFF/APRIL antibody for systemic lupus erythematosus; and TST808, a biparatopic anti-APRIL antibody for IgAN.
The manufacturing costs for biologics can be high and the scale-up process complex, which increases the costs of the final therapeutics.
“We wanted to create innovative and accessible therapeutics, especially in areas of China where affordability are low, and so we developed our Highly Integrated Continuous Bioprocessing [HiCB] manufacturing platform. This integrates developed in-house continuous upstream-perfusion process with an automated and continuous downstream process co-developed with Merck KGaA,” said Qian.
Transcenta is working with partners to commercialize its therapeutics worldwide. “We are in discussion with potential partners for osemitamab for gastro-intestinal tract indications. All of our pipeline therapeutics are available for co-development or licensing, and we are interested in bringing in innovative technologies and assets to add to our portfolio,” said Qian.
Transcenta also provides contract development and manufacturing services, as well as access to its bioprocessing technology and knowhow.

Accessible Antibody Therapeutics for Cancer and Autoimmune Diseases

In Conversation | Xueming Qian – CEO & Co-Founder, Transcenta

Putting Biologics on the Fast Lane

Industrialization of Highly Intensified Connected/Continuous Biomanufacturing Platform (HiCB) to Address Patient and Business Needs

A CTO's Journey Into Continuous Biomanufacturing

TST001 Under Evaluation in Locally Advanced or Metastatic Solid Tumors