TRANSCENTA HOLDING - A Global Fully Integrated Biotherapeutics Company
Transcenta, Biologics, Antibody, Claudin 18.2


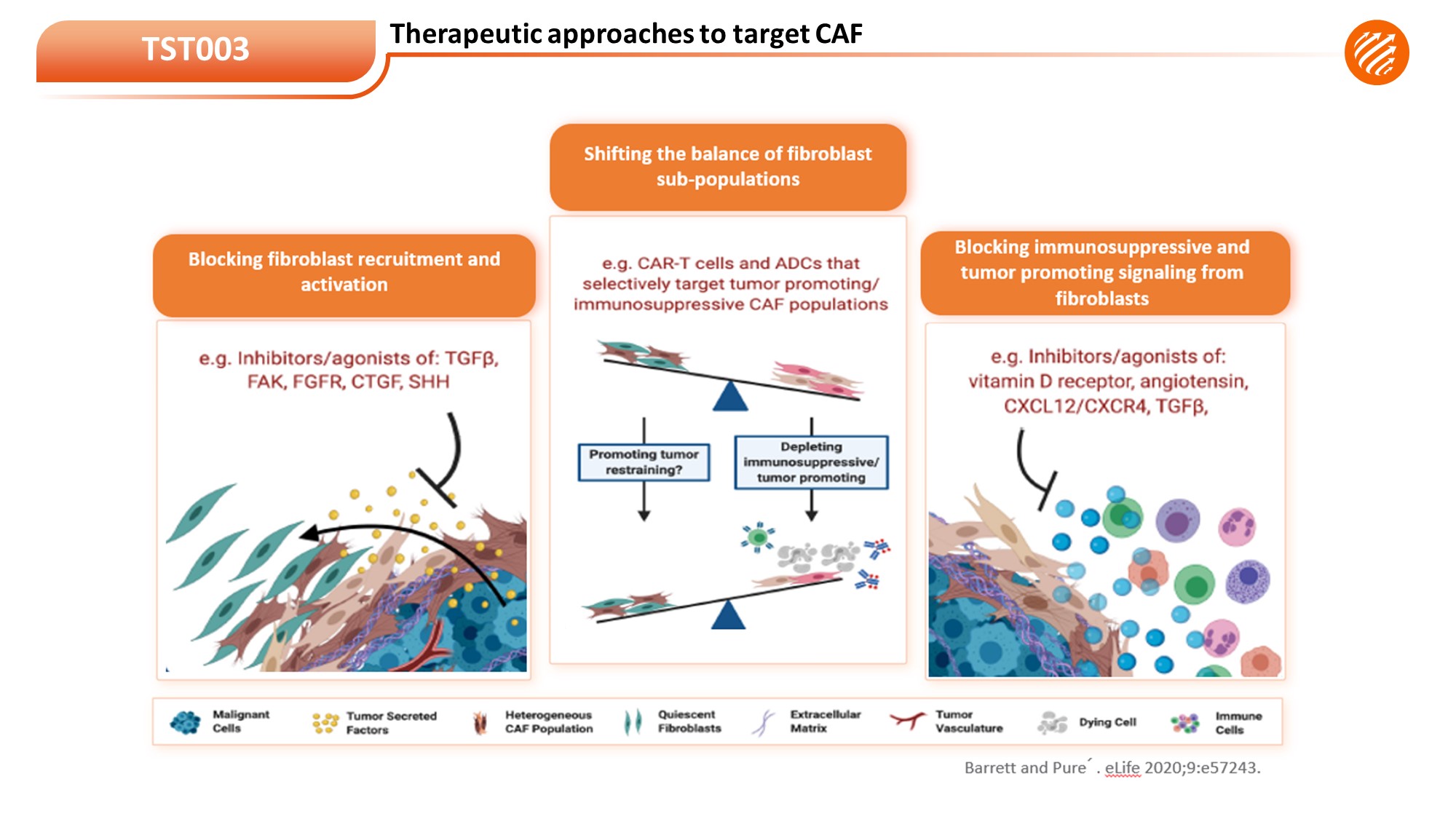
TST003 is a first-in-class and high affinity humanized monoclonal antibody targeting GREMLIN-1, a regulatory protein that is highly expressed by stromal cells and tumor cells in diverse human carcinomas, especially in colon cancer, prostate cancer, gastric cancer, lung cancer, esophageal cancer, pancreatic ductal adenocarcinoma, and breast cancer. It is currently tested in a global FIH trial.
• TST003-1001 study, the FIH trial, is ongoing at multiple clinical centers in the U.S. and China. Dose escalation as monotherapy has been completed. TST003 has demonstrated good safety and tolerability, and dose proportional PK profiles were observed.
• A Trial in Progress (TiP) poster of TST003-1001 study was presented at the 2024 AACR annual meeting.
• First-In-Class Gremlin1 Targeting Antibody
• Target highly expressed in stromal cells in tumor microenvironment
• Targeting CPI resistant/ineligible solid tumors including Prostate cancer, NSCLC, CRC, ESCC, GC, PADC, Breast Cancer et
Academic Collaboration
Researchers from Transcenta and Shanghai Jiao Tong University investigated the therapeutic potential of anti-Gremlin1 for prostate cancer in an aggressive mouse model of CRPC (Pbsn-Cre4; Ptenfl/fl; Trp53fl/fl GEMM) and patient derived xenograft (PDX), using TST003, a humanized Gremlin1-targeting antibody, and a surrogate antibody generated by Transcenta.

Mar, 2023
Jan, 2023
Sep, 2022
May, 2022